Global Site Management & Clinical Trial Support Solutions
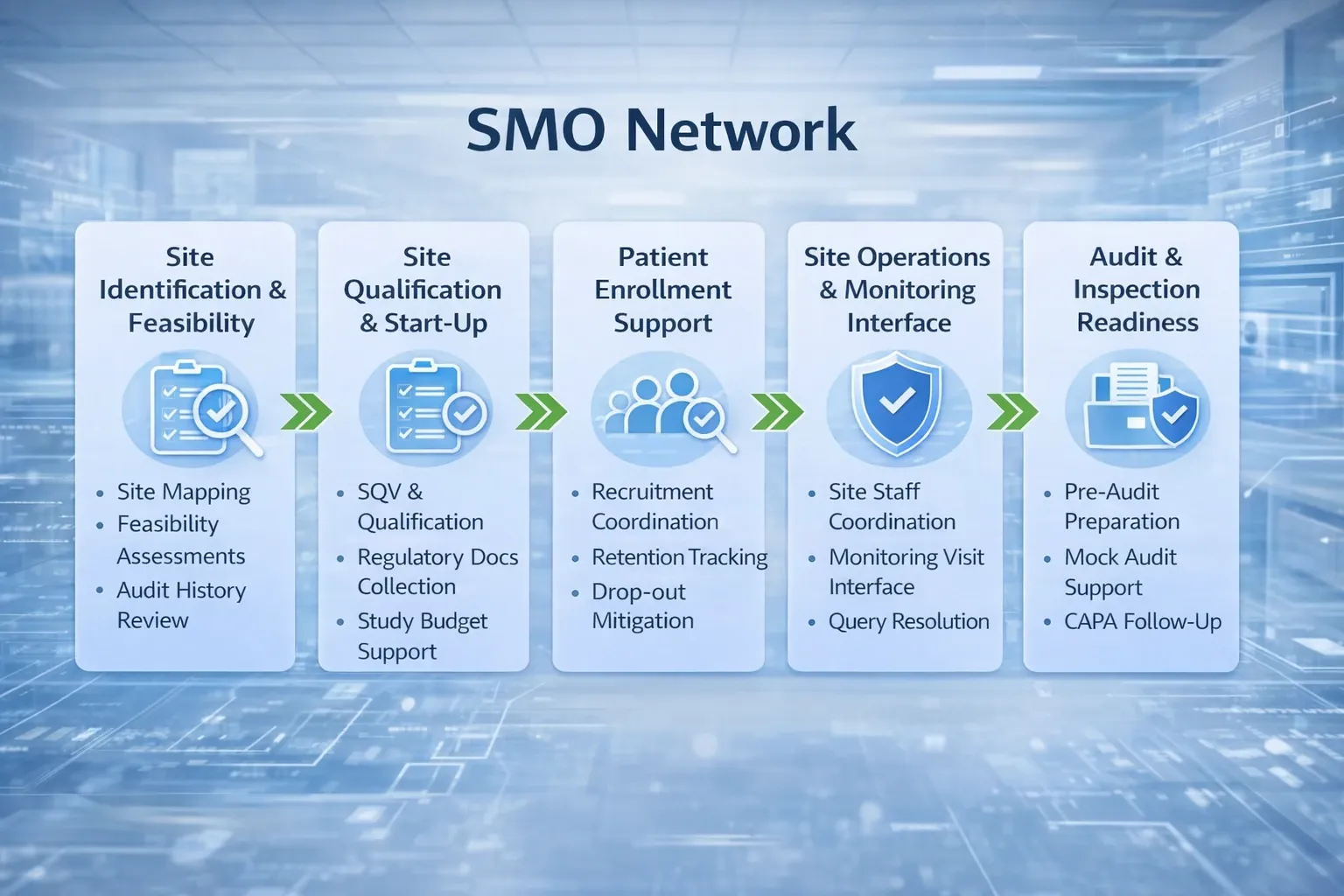
At Bosker Medico, our Site Management Organization (SMO) division bridges the gap between clinical trial sponsors and investigative sites. We manage the trial lifecycle—from feasibility to closeout—with a focus on CDSCO and NDCT 2019 regulatory excellence.
By integrating ICH-GCP standards with local expertise in the New Drugs and Clinical Trials (NDCT) Rules, 2019, we ensure high-quality data and inspection-readiness. Our SMO model accelerates "First Patient In" (FPI) timelines through a robust network of GCP-trained investigators.
Driving Efficiency at the Investigative Site
Specialized Therapeutic Area Expertise
High-Complexity & Specialty
Emerging & General Medicine
The Bosker Medico SMO Network in Numbers
10+
Active Sites
10+
Audits Cleared
30+
Pharma Clients
10k+
Patient Enrolments