Proactive Safety: The Sentinel Approach to Signal Detection
In the landscape of Indian Pharmacovigilance, identifying safety signals before they escalate into regulatory concerns is the hallmark of a pioneer. Bosker Medico Services utilizes advanced data validation to ensure GVP-compliant risk management for global pharmaceutical partners.
The Pharmacovigilance Signal & Risk Management Lifecycle
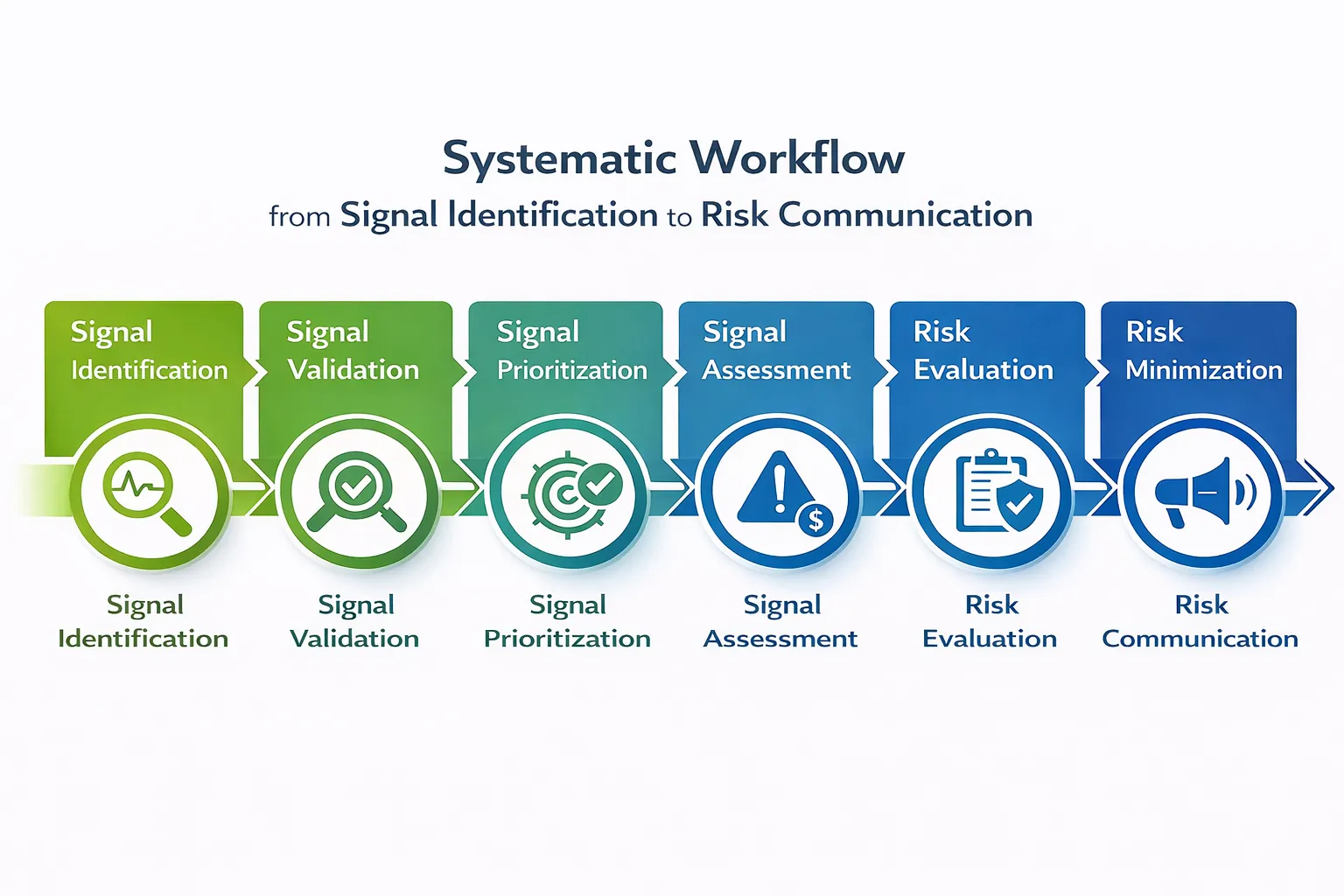
Figure 1: Systematic workflow from Signal Identification to Risk Minimization.
We monitor multiple data streams to detect new or changing causal associations between interventions and adverse events, ensuring patient safety across all therapeutic areas in India through rigorous validation and assessment.
Aggregate Reporting (PBRER)
Compiling high-fidelity Periodic Benefit-Risk Evaluation Reports that meet stringent CDSCO and global health authority (FDA/EMA) requirements. Our reports offer a holistic view of the molecule's safety profile.
Risk Management (RMP)
Developing specialized Risk Management Plans and minimization measures to navigate complex safety profiles under GVP Module V, ensuring continuous compliance and market access.
Why Bosker Medico is a Pioneer in Indian PV
Achieve Unmatched Safety Compliance
Leverage our expertise as a leading PV sentinel in the Indian market.
Request a Technical Consultation