Navigating the Journey: Clinical Trial Phases in India
In the evolving landscape of Indian drug development, clinical trials serve as the scientific bedrock for evaluating safety and efficacy. At Bosker Medico, we view these studies through the lens of the New Drugs and Clinical Trials (NDCT) Rules, 2019, ensuring every phase is a GVP and GCP-compliant asset.
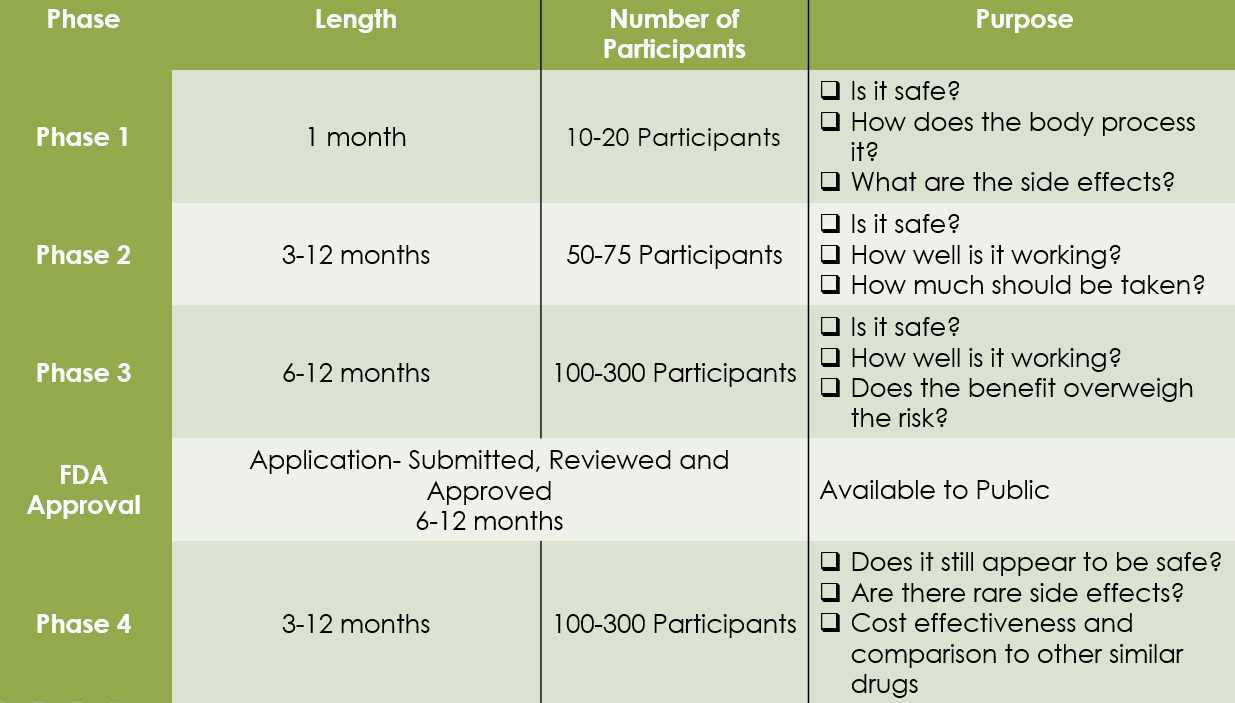
Phase I: Human Pharmacology
The first step in human testing. Our focus is on Pharmacokinetics (PK) and Pharmacodynamics (PD). We help sponsors establish the initial safety profile and dose-escalation logic required for regulatory dossiers.
Phase II: Therapeutic Exploratory
Conducted in a larger patient cohort to evaluate preliminary efficacy. We provide Specialized SMO support to ensure rapid recruitment and high-fidelity data collection for specific indications.
Phase III: Therapeutic Confirmatory
Global multi-center trials involving thousands of participants. Bosker Medico ensures Zero-Critical-Observation readiness for CDSCO, US-FDA, and EMA inspections through rigorous site management.
Phase IV: Post-Marketing Surveillance
Crucial for market longevity. Our Pharmacovigilance (PV) sentinel monitors rare adverse effects and long-term benefit-risk profiles, ensuring compliance with global safety mandates.
The Clinical Research Lifecycle
Accelerate Your Clinical Narrative
Looking for a specialized Indian partner to manage your trial phases with regulatory precision and scientific integrity?
Consult Our Experts