Comprehensive Drug Safety & PV CRO Solutions

Bosker Medico provides end-to-end Pharmacovigilance (PV) services and drug safety solutions as a premier Contract Research Organization (CRO).
We ensure proactive safety monitoring and strict regulatory compliance across the product lifecycle—covering early-phase clinical trials through to global post-marketing surveillance and risk management.
End-to-End Drug Safety Capabilities
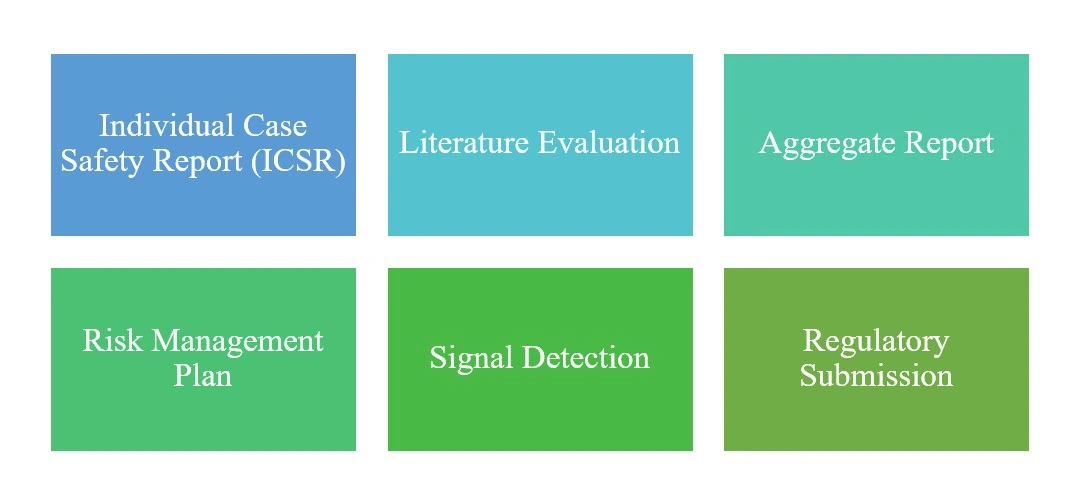
Our dedicated team of safety experts ensures GVP (Good Pharmacovigilance Practices) compliance, utilizing industry-leading safety databases to navigate complex global regulatory landscapes for pharmaceutical and biotech clients.
Individual Case Safety Report (ICSR) Processing
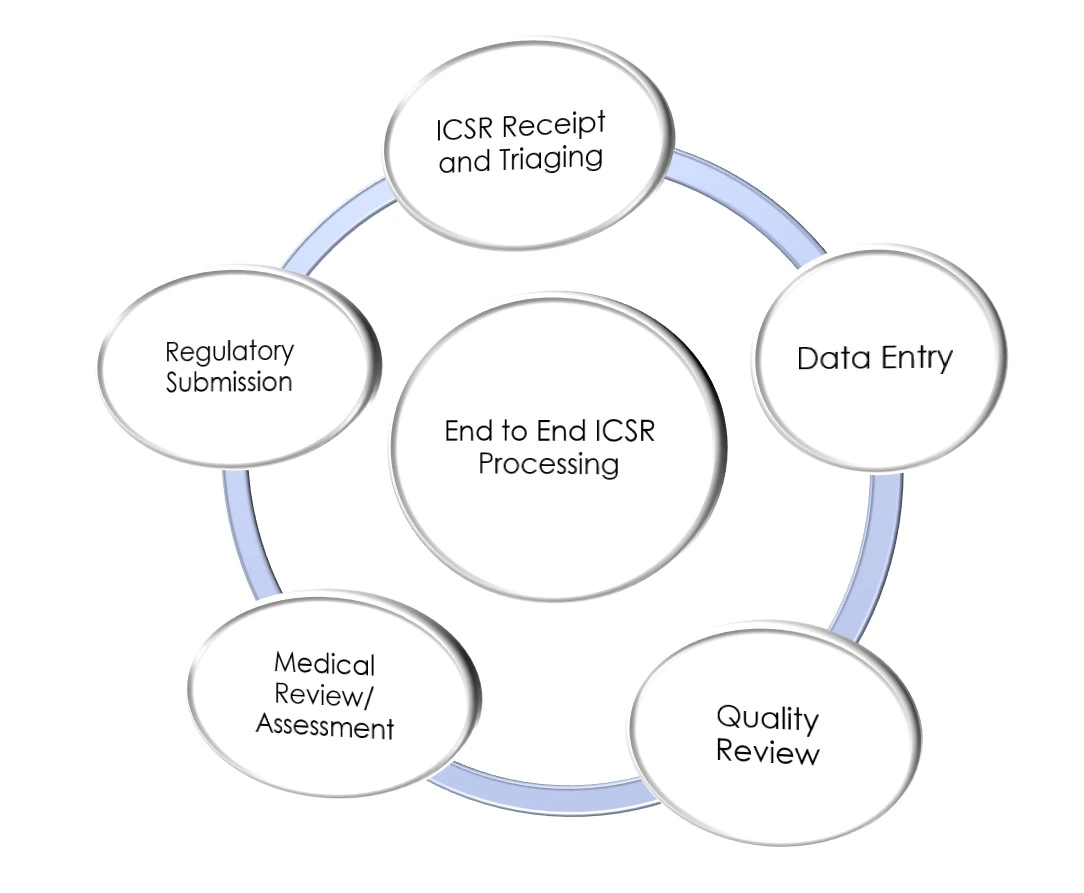
We manage the full lifecycle of safety cases, including triaging, high-volume data entry, MedDRA coding, and rigorous quality review. Our medical review process guarantees precision in safety reporting for spontaneous, solicited, and clinical trial data.
Aggregate Safety Report Writing (PBRER/PSUR)
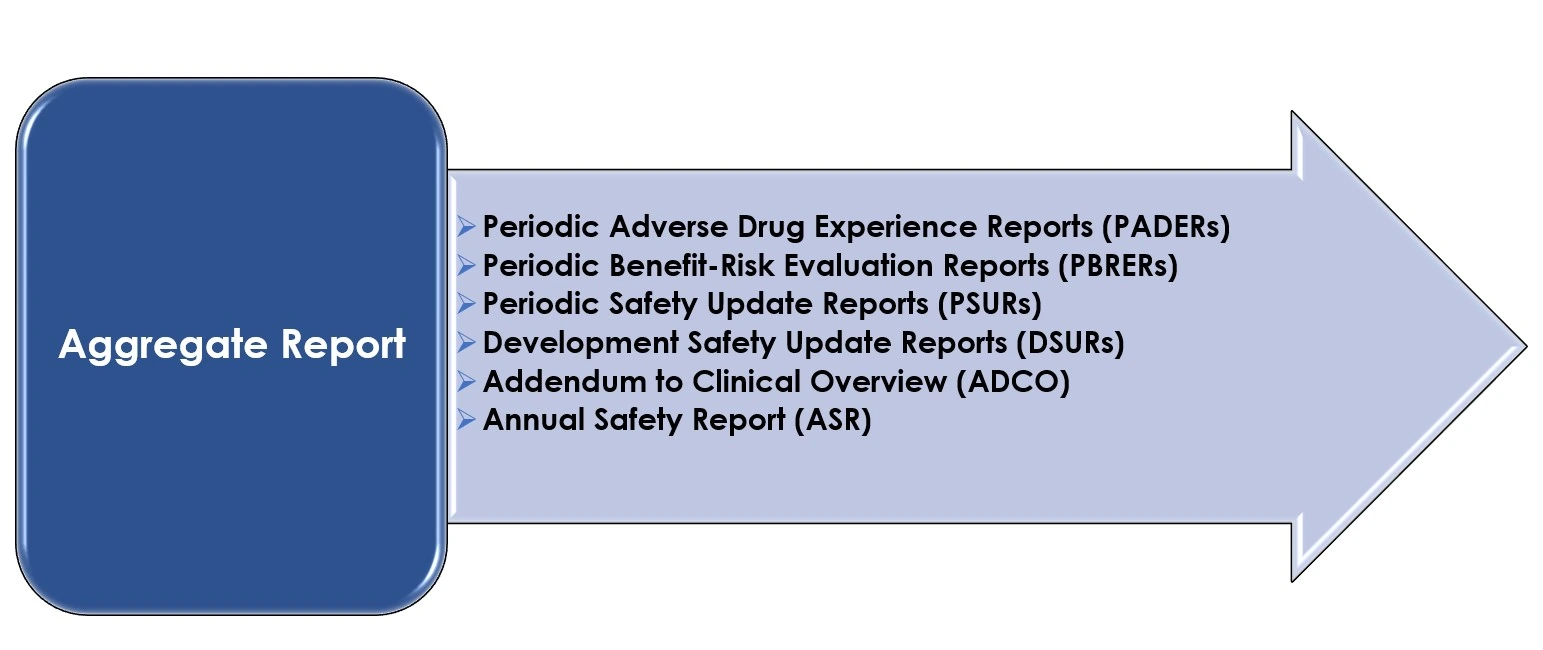
Our expert medical writers specialize in preparing global periodic safety reports including PBRER, PSUR, and DSUR. We provide evidence-based benefit-risk assessments tailored to meet USFDA, EMA, and MHRA submission standards.
Pharmacovigilance Signal Detection & Analytics
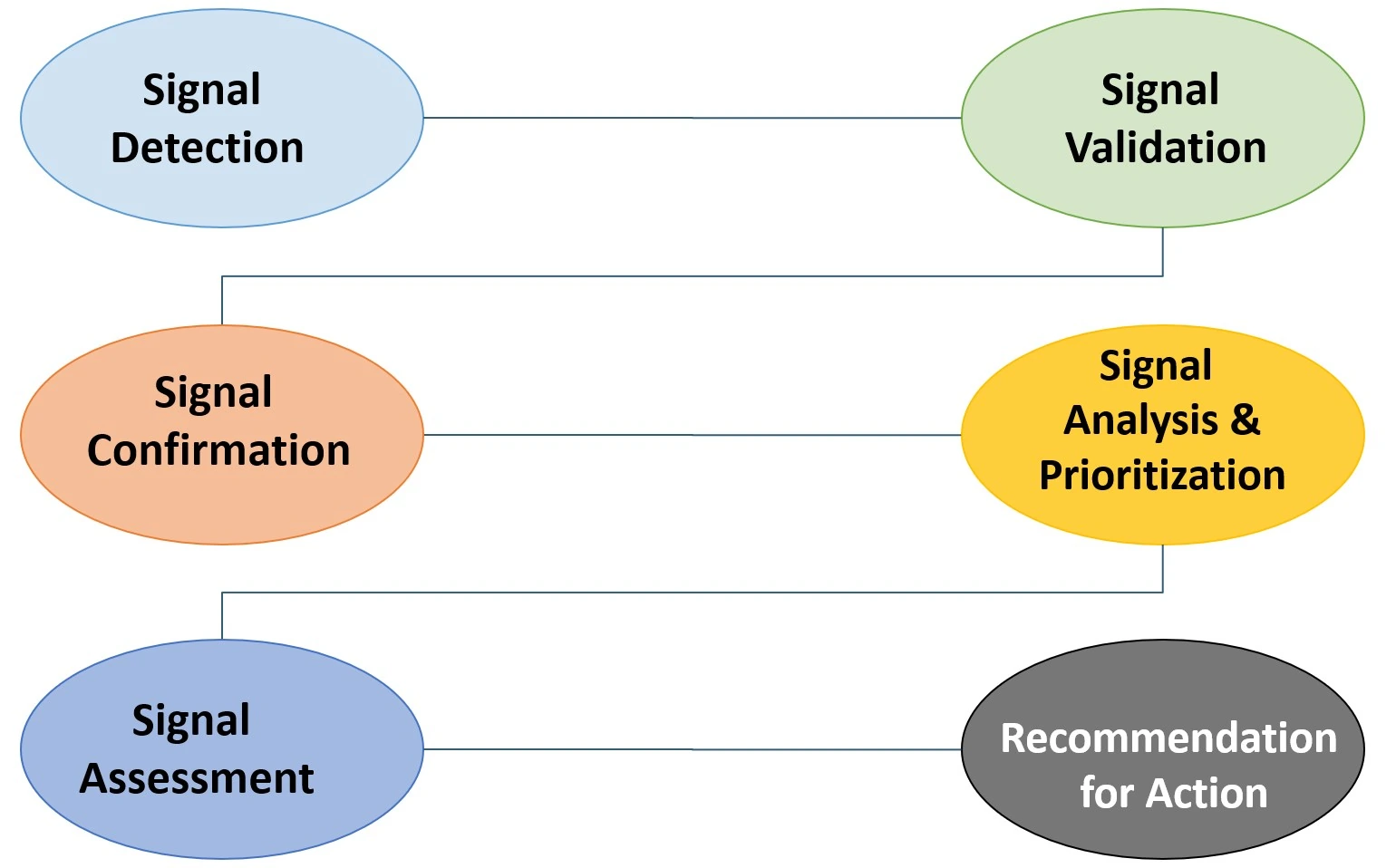
Continuous safety profile monitoring to identify and validate potential signals. Using advanced statistical methodologies, we ensure the ongoing safety, efficacy, and compliance of your medicinal products in the market.
Stay ahead of regulatory changes: Read our latest analysis on Pharmacovigilance India 2026 Trends and download the audit checklist.