Global Regulatory & Scientific Publication Solutions

Bosker Medico is a premier life-sciences partner providing high-impact Medical and Scientific Writing services tailored for global pharmaceutical, biotechnology, and medical device companies.
Our expert medical writers are specialists in ICH-GCP, EMA, and USFDA guidelines. We ensure the creation of precise, submission-ready clinical and regulatory documentation that streamlines the path to marketing authorization.
Comprehensive Scientific Writing Capabilities
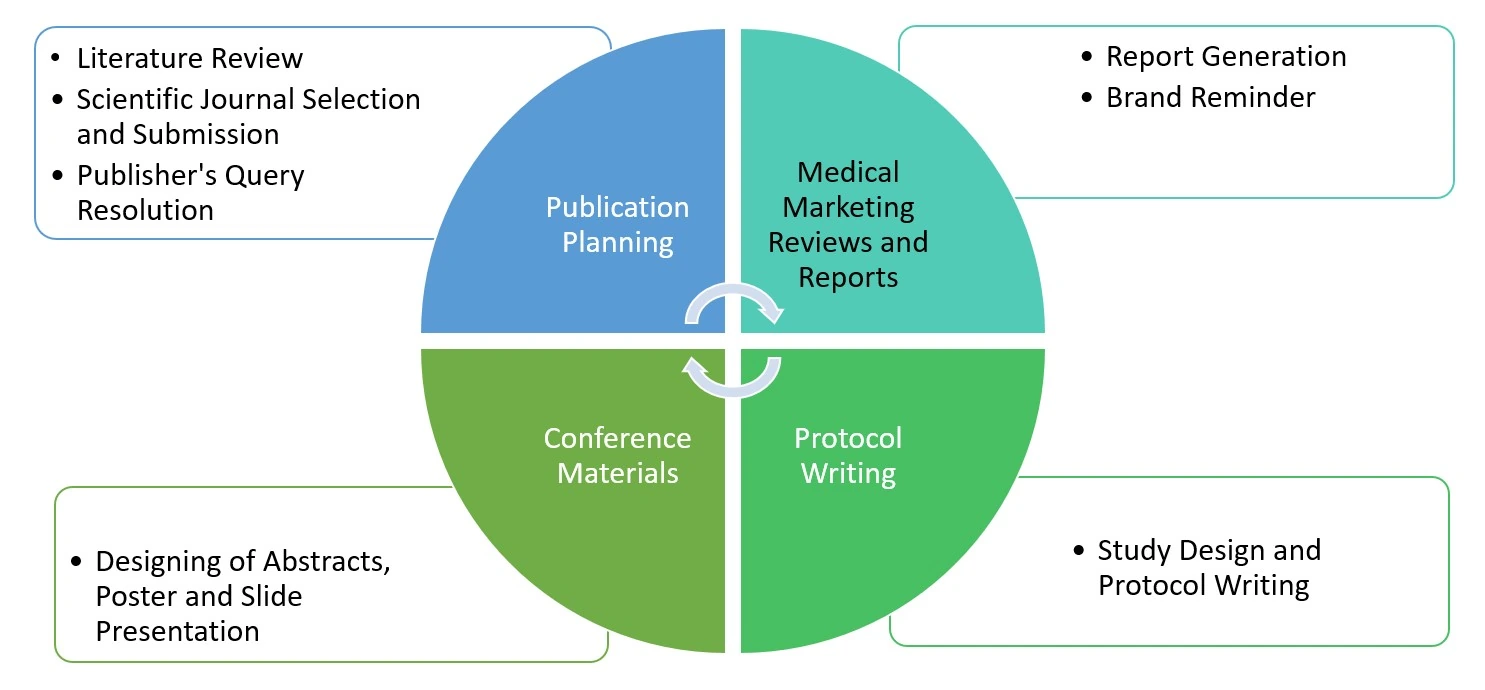
Regulatory Writing: Clinical & Non-clinical
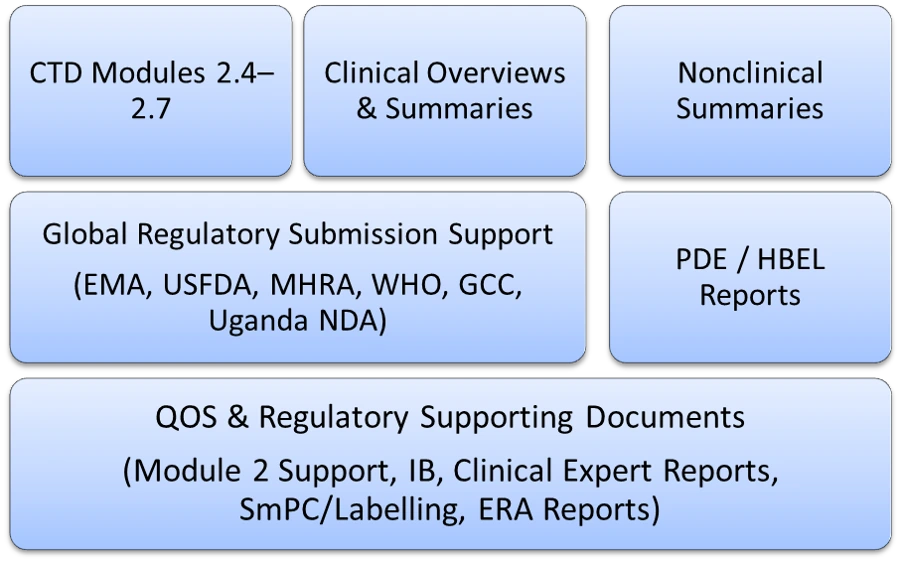
We transform raw clinical data into a compelling regulatory narrative. Our writers ensure that data is scientifically interpreted to meet Common Technical Document (CTD) standards.
Environmental Risk Assessment (ERA)
We provide scientific transparency and compliance for aquatic, terrestrial, and microbial impact assessments, ensuring lifecycle support for post-approval submissions.