Strategic Clinical Research & Computer System Validation (CSV)

At Bosker Medico, our Clinical Research and CSV solutions are engineered for pharmaceutical excellence, ensuring absolute data integrity and regulatory precision. We validate your digital ecosystem to meet GAMP 5 and global GxP standards.
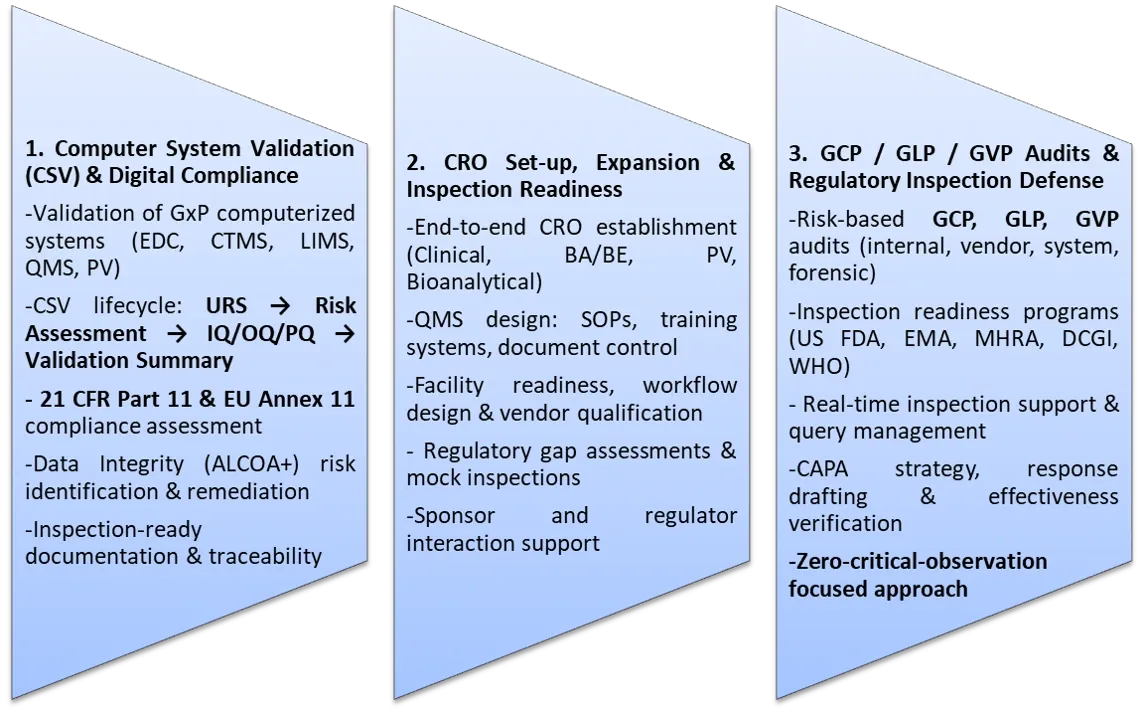
1. Computer System Validation (CSV) & Digital Compliance
2. CRO Set-up, Expansion & Inspection Readiness
We facilitate global CRO expansion across Clinical and BA/BE domains with a focus on sustainable quality infrastructure.
3. GCP / GLP / GVP Audits & Inspection Defense
Our zero-critical-observation focused approach prepares your organization for the world's most stringent health authorities.
The Bosker Medico Clinical Research Shield
We provide a comprehensive technical framework to protect your clinical and validation data, ensuring 100% audit-readiness.
Secure Your Data Integrity Today
Leverage our CSV and Clinical Research expertise to ensure zero-critical-observation success.
Speak to a Compliance Consultant